Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Scientific Reports volume 13, Article number: 21824 (2023 ) Cite this article Blood Tube Prp

Ulcerative keratitis is a common disease in horses which may cause blindness. To prevent secondary bacterial and fungal infections and promote quick re-growth of the epithelial layer, different treatment approaches have been employed. This study aimed to examine the effects of advanced platelet-rich fibrin (A-PRF) gel on the healing process of experimentally induced corneal ulcers in donkeys. Nine healthy adult donkeys were used for the study. The donkeys were divided into two groups: the control group, where no medication was applied to the corneal ulcer, and the A-PRF gel group, where A-PRF gel was applied once a day on specific days after ulcer induction. The healing process was evaluated through various examinations and analyses. The results demonstrated that the A-PRF gel group showed significant improvement in the corneal ulcer area, with epithelial and stromal regeneration. At day 35, about 60% of the A-PRF group showed negative fluorescein uptake. Additionally, fewer complications were observed during the healing process compared to the control group. In conclusion, A-PRF gel is an important and safe therapeutic option for controlling ocular surface infection and promoting corneal healing. We recommend using A-PRF gel as an alternative approach, avoiding eyelid suturing, and minimizing corneal irritation.
Chanatip Metheetrairut, Panotsom Ngowyutagon, … Pinnita Prabhasawat
Dong Ju Kim, Mi-Young Jung, … Choul Yong Park
Takehiro Matsumura, Kentaro Iwasaki, … Masaru Inatani
Corneal ulcers are a significant concern in the equine industry and for veterinarians due to the unique anatomy of horse's eyes1,2,3,4,5. Ulcerative keratitis, characterized by inflammation and damage to the cornea, can occur as a result of infections caused by microorganisms from the environment or the ocular surface. These infections can lead to the infiltration of immune cells and the production of enzymes that can cause the cornea to melt1,6,7. Complex corneal ulcers, which involve deep layers of the cornea and are often associated with infection, are particularly challenging8,9.
Various treatment approaches have been used to prevent secondary infections and promote rapid healing of corneal ulcers, but the optimal drug therapy is still uncertain1. Medical therapy is often the first choice, as differentiation between simple superficial corneal erosions and superficial non-healing corneal ulcers may be difficult at the onset of ocular problems. The medical treatments generally give disappointing results with superficial non-healing corneal ulcers while the absence of response after a while may be a sign of the presence of such ulcers10. The goals of medical therapy for superficial corneal ulcers are to reduce contamination and prevent bacterial colonization of the exposed corneal stroma until re-epithelialization has occurred11. A soft contact lens can be used to protect the cornea. In an animal that was medically treated for an indolent ulcer, the affected eye became significantly less painful as soon as the soft contact lens was applied. The contact lens allowed the animal to undergo training during the time of treatment. When loosened epithelial margins are present, debridement is advised using a cotton tip or small curette12. Superficial debridement of the cornea (Keratectomy) in conjunction with temporary tarsorrhaphy, third eyelid flap, or conjunctival flap, can be used with unmedical treatable cases13. Recent advancements in regenerative therapies aim to restore damaged tissues and address ophthalmological conditions14. One of the recent interesting regenerative therapies is amniotic membrane transplantation (AMT) and the use of recombinant growth factors. However, the results obtained after the application of AMT, but the costly manufacturing, and the seldom clinical results, make it obligatory to find other therapeutic ways for corneal regeneration15,16,17,18. Matrix regenerating agents (RGTA) are recent topical agents that show encouraging results in promoting tissue healing and regeneration, including cases of neurotrophic ulcers, corneal dystrophies, or postsurgical conditions19,20. Recently, drops coming from blood derivatives have also been described as a treatment for ophthalmology disorders, including persistent corneal epithelial defects18. Platelet concentrates, derived from a patient's blood, contain growth factors, fibrin, and sometimes leukocytes, which are collected and processed into a clinically functional form21,22. These concentrates can be in liquid form for distillation or injection, or gel form for application to wounds or injured areas to promote tissue regeneration22,23,24,25. Recently, a complete classification of platelet concentrate technologies depending on their leukocyte content and fibrin architecture has been proposed26. One specific type of platelet concentrate is platelet-rich fibrin (PRF), which is an autologous fibrin-based membrane, matrix, or scaffold 21,25,27,28,29,30.
The impact of using autologous advanced platelet-rich fibrin gel on the recovery of artificially-induced corneal ulcers in donkeys (Equus asinus).
This study was conducted as a brief prospective investigation at the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, specifically in the Department of Surgery, Anesthesiology, and Radiology. The procedures of this study were approved by the National Ethics Committee of the Faculty of Veterinary Medicine, University of Assiut, Assiut, Egypt, in accordance with both Egyptian law and the Animal Welfare Standards for the Care and Use of Animals in Research and Education set by the OIE. The research was carried out in adherence to the guidelines and regulations outlined in the ARRIVE guidelines (https://arriveguidelines.org).
The study involved a total of nine donkeys (Equus asinus), consisting of four males and five non-pregnant, non-lactating females. The donkeys were selected based on their age, ranging from 7 to 8 years with an average of 7.4 years, and their body weights, ranging from 100 to 130 kg with an average of 115 kg. Prior to the study, the donkeys underwent an eye examination to ensure they had no pre-existing eye deformities. Throughout the study, the donkeys were housed in well-equipped stalls and provided with a balanced ration twice a day, along with access to green forage. They were also given unrestricted access to water.
The eyes were assessed for their normal shape, presence of any discharge, position and movement of the eyeballs, reflexes of the eyelids and cornea, as well as the threat response. In a dimly lit room, the pupil's response to light, condition of the eyelids, nictitans, conjunctiva (both bulbar and palpebral), cornea, iris, and lens were thoroughly examined31.
To sedate the animals, they were given an intravenous (IV) injection of 1.1 mg/kg xylazine hydrochloride 2% (Xyla-Ject, ADWIA Co., SAE, Egypt) and 2.2 mg/kg ketamine hydrochloride 5% (Ketamine, Sigma- tec Pharmaceutical Industries, SAE, Egypt). Additionally, three drops of benoxinate hydrochloride 0.4% (Benox, Sterile Ophthalmic Solution, Egyptian Int. Pharmaceutical Industries Co., Egypt) were applied to the corneal surface as a surface analgesia.
Method of inducing corneal ulcers; a cotton swab dipped in 1N NaOH was applied to the corneal surface, after using a sterile stainless steel washer as a template (A), a central corneal ulcer was now induced and X-rayed with a ruler as a yardstick (B) and fluorescein staining of the ulcer (C). Preparation and application of the A-PRF gel; the A-PRF gel was formed in the Vacutainers after centrifugation (D), the gel was then brought out with sterile forceps (E), the finished gel after cutting with scissors (F), stained smear of the A-PRF gel examined by a light microscope (G), the A-PRF gel was applied to the corneal surface after ulcer induction (H), and two interrupted skin sutures were placed to hold the gel in place (I).
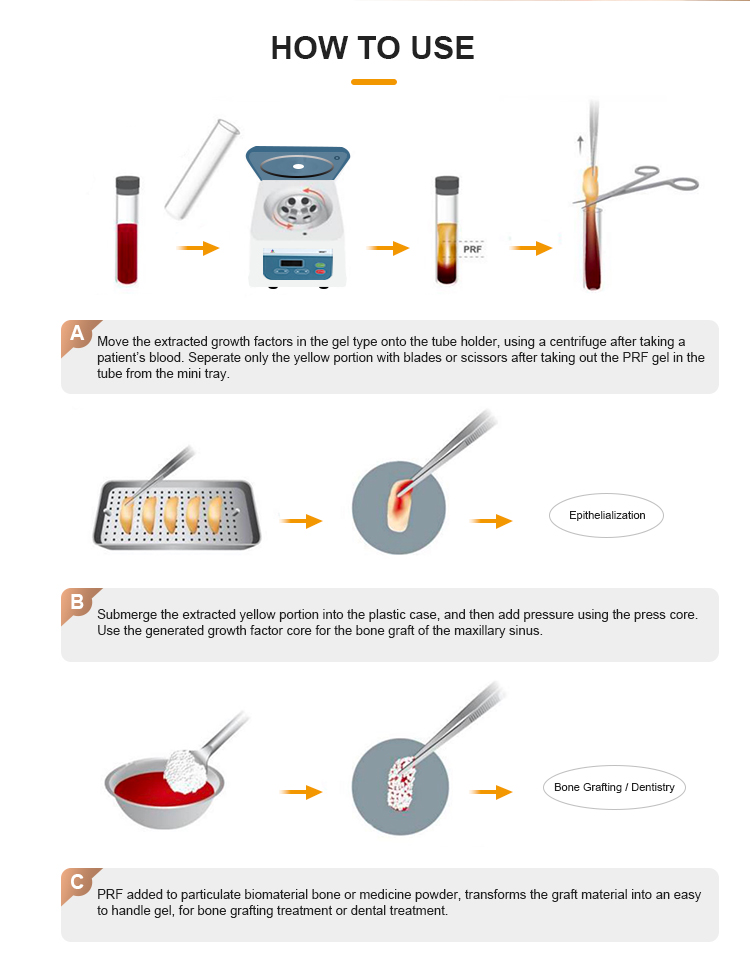
The donkey was positioned on its side after anesthesia, with the operated eye placed on top. To induce a centric corneal ulcer with a diameter of 6 mm, a solution of 1N sodium hydroxide (NaOH) was used32,33. A sterile cotton swab with a diameter of 4 mm was soaked in the NaOH solution for 5 s. The eye was opened using an eyelid dilator, and the swab was placed on the corneal surface for 60 s34. To ensure a uniform circular ulcer, a sterile stainless steel washer with a 6 mm inner diameter was used as a template. The excess NaOH was then rinsed off by irritating the eye with 20 mL of physiological saline35. The donkeys were randomly divided into two main groups: the control group (A) consisting of three donkeys, where no drugs were applied to the corneal ulcer throughout the study, and the advanced platelet-rich fibrin gel (A-PRF gel) group (B) consisting of six donkeys, where A-PRF gel volume which obtained from centrifugation of 10 ml blood of own animal without any anticoagulants was applied topically over the cornea (in the cal de sac) once daily on days 0, 2, 4, 6, 9, 13, 20 , and 27 after corneal ulcer induction. To ensure the gel remained in contact with the corneal surface, the lid fissure was closed using two interrupted horizontal silk sutures (#0) that went through the skin of the upper and lower eyelids (skin to skin). This was done to keep the A-PRF gel in place (see Fig. 1).
A 10 ml sample of the donkey's own venous blood was collected from the jugular vein using a sterile 10 ml syringe and an 18G1.1/2 hypodermic needle. The blood was immediately placed in a sterile glass-based vacuum tube. The tube was then centrifuged at 1500 rpm for 14 min using a CENTRIFUGE PLC SERIES, Model: PLC-05, SN.818555, Gemmy Industrial Corp., Taiwan. This process resulted in the formation of three layers: platelet-poor plasma (PPP) on the top, red blood cells at the bottom, and the A-PRF gel or clot in the middle32,36. To use the A-PRF gel, the clot was carefully removed from the tube using sterile forceps, and the layer of red blood cells was trimmed away with sterile scissors to obtain a clean A-PRF gel.
All animals were closely monitored and assessed on specific days (2, 4, 6, 9, 13, 20, 27, and 35) after the corneal ulcers were induced. To conduct eye examinations and capture photographs, the animals were sedated using an intravenous injection of 1.1 mg/kg xylazine hydrochloride 2%. Additionally, retrobulbar and auriculo-papebral nerve blocks were performed using 20 ml and 5 ml of 2% lidocaine hydrochloride, respectively. The evaluation process included clinical and external ophthalmologic examinations, fluorescein staining, assessment of ulcer healing, and histopathology.
The donkeys were observed and evaluated for their activity levels, resumption of eating and drinking, and signs of pain for a period of 3–5 days after waking up from anesthesia. Physiological measurements such as body temperature (BT), heart rate (HR), and respiratory rate (RR) were recorded both before the surgery and after the donkeys had fully recovered from anesthesia.
The cornea and conjunctiva were visually inspected for signs of swelling, excessive tearing, discharge, and eyelid spasms. Using a binocular loupe and a focused light source, the presence of corneal opacity, neovascularization, pigmentation, and conjunctivitis were also examined. These assessments were conducted on days 2, 4, 6, 9, 13, 20, 27, and 35 following the induction of corneal ulcers. Additionally, the healing progress of the ulcers and the formation of scar tissue were evaluated.
To assess the presence of corneal ulceration, a 2% fluorescein solution was applied to the eye on days 2, 4, 6, 9, 13, 20, 27, and 35. The solution was left in the eye for one minute before being flushed out with sterile normal saline. The eye was then examined for any remaining stain using a pen light and binocular loupe37.
Photographs of the eye were taken twice during the study: once after conducting an external eye examination and again after fluorescein staining. These photographs were captured on days 2, 4, 6, 9, 13, 20, 27, and 35 using a digital camera. Prior to capturing the images, the eye was flushed with normal saline, and a standard ruler was placed as close as possible to the ulcer. To assess the rate of ulcer healing, the surface areas of the corneal ulcer were measured in square centimeters using Image J software (version 4.48v, National Institutes of Health, USA) and analyzed32.
Histopathological examination was performed after donkeys were treated according to the protocol38 by intravenous injection of xylazine hydrochloride (1.1 mg/kg) followed by rapid intravenous injection of thiopental sodium (thiopental sodium 1 g vial, EPICO, Egypt) at a dose of 35 mg/kg; the corneas of the donkeys were collected and fixed in 10% neutral buffered formalin. The fixed samples were then dehydrated, clarified, and embedded in paraffin wax. Thin sections (5 µm) were cut from the paraffin blocks and stained using various histological techniques. Hematoxylin and eosin (H&E) stain was used for general histological examination39, periodic acid-Schiff (PAS) technique was employed to detect neutral mucopolysaccharides40, Crossmons trichrome technique was used to stain corneal collagen fibers41, and Picro-Sirius red technique was utilized to differentiate between mature and immature corneal collagen42. The stained sections were observed under an Olympus BX51 microscope, and images were captured using an Olympus DP72 camera attached to the microscope.
The data were analyzed using the GLM procedure of SAS (SAS Institute, 2009) and expressed as mean ± SE. Significant treatment effects were determined using ANOVA, and differences between the least squares means were assessed using Duncan's multiple range test. Statistical significance was considered at a level of P < 0.05.
On the day of ulcer induction, the donkeys exhibited signs of pain such as restlessness, head droop, isolation, and decreased food intake. However, by the fourth day after ulcer induction, most of the donkeys had returned to normal food consumption and the head droop had resolved in all but two of them. These two donkeys continued to show head droop and decreased activity until the fourth postoperative day. However, by the fifth day of the study, all donkeys had returned to normal activity and performance. The physiological parameters, including heart rate (HR), respiratory rate (RR), and body temperature (BT), remained within the normal range throughout the study, and there was no significant difference in these parameters before and after ulcer induction (Table 1).
Serial photos of the follow-up of the induced corneal ulceration in the control and A-PRF gel group at days 0, 6, 13, 27 and 35 in donkeys.
From the second day after ulcer induction until day 35, blepharospasm (eye twitching) was observed in all donkeys except one, which resolved by day 20. Epiphora (excessive tearing) and corneal opacity were present on the second day after ulcer induction and gradually decreased from day 20 to day 35. Corneal vascularization (formation of new blood vessels) varied in severity, with some donkeys showing superficial neovascularization and others showing deep neovascularization. Overall, corneal vascularization decreased gradually from day 20 to day 35. Two donkeys in the control group had associated conjunctivitis, which resolved in one donkey by day 13.
Blepharospasm and epiphora were observed in all donkeys in this group, except for two donkeys, from day 2 until the end of the study (day 35). Eyelid swelling started on the second day of ulceration and resolved on the ninth day in three donkeys, and on the 27th day in the other three donkeys. Corneal vascularization varied in severity, with some donkeys showing superficial neovascularization and others showing deep neovascularization. Neovascularization peaked on day 13 and started on day 2 in four animals and on day 4 in two animals. Conjunctival congestion was present in all animals from the second day of the study and began to resolve on the ninth day in two animals and the 20th day in four animals.
Serial photos of follow-up of induced corneal ulceration after fluorescein staining in control and A-PRF gel group at days 0, 6, 13, 27 and 35 in donkeys.
In the control group (A), two donkeys showed a disappearance of fluorescein uptake on day 20, while one donkey showed a disappearance on day 35. In the A-PRF gel group (B), four donkeys had a disappearance of fluorescein uptake on day 35, one donkey on day 9, and one donkey had a reuptake on day 6 followed by a disappearance on day 9 and again on day 13.
In group (A), the average size of the ulcer (measured in cm2) at day 35 was significantly reduced (P < 0.041) compared to the initial measurement at day zero (Table 2, Fig. 4). Similarly, in group (B), the average ulcer size at days 2, 4, 6, 9, 13, 20, 27, and 35 showed a significant decrease (P < 0.0001) compared to the initial measurement at day zero (Table 2, Fig. 4). The results of image analysis after fluorescein staining also indicated a significant decrease in the average ulcer size at days 4, 6, 9, 13, 20, 27, and 35 compared to day zero in both group (A) and group (B) (P < 0.0001) (Table 2, Fig. 4).
Mean (SE) surface area (in cm2) of corneal ulceration without fluorescein (top) and with fluorescein (bottom) in the control group (n = 3) and the A-PRF gel group (n = 6).
During the study, several complications were observed (Fig. 5). These included pigment keratitis, which was seen in one donkey from group (A) and four donkeys from group (B) starting from day 20 until the end of the study. Additionally, one donkey from the group (B) developed anterior synechia on day 20.
Complications recorded during the follow-up period of induced corneal ulceration in donkeys; eyelid swelling and blepharospasm (A), epiphora (B), anterior synechia (C), pigmented keratitis (D), pigmented keratitis with scarring (E).
The negative control group exhibited a normal histological structure of the cornea, including the corneal epithelium, Bowman's membrane, corneal stroma, Descemet's membrane, and corneal endothelium (Figs. 6a, 7a, 8a, 9a, 10a and 11a).
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea. (b) Day 0 group with induced corneal ulcer showing epithelial defect and edema in the corneal stroma. (c) The positive control group shows regenerated corneal epithelium, subepithelial blood coagulation, edema, leukocyte infiltration (L), vascular congestion and neovascularization in the corneal stroma. (d) The PRF gel panel shows regenerated corneal epithelium and neovascularization in the corneal stroma. Original enlargement; ×100, scale bar = 200 µm, hematoxylin and eosin stain.
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea. (b) Day 0 group with induced corneal ulcer showing an epithelial defect, dispersed collagen bundles and edema in the corneal stroma. (c) The positive control group shows regenerated corneal epithelium and dispersed collagen bundles, neovascularization, edema in the corneal stroma. (d) The PRF gel group shows regenerated corneal epithelium and regularly dense collagen bundles, edema in the corneal stroma. Original enlargement; ×400, scale bar = 50 µm, hematoxylin and eosin stain.
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea; numerous keratocytes between the dense regular collagen bundles. (b) Group with induced corneal ulcer on day 0 showing numerous keratocytes between the collagen bundles. (c) The positive control group showing numerous fibroblasts between the irregular dense collagen bundles, neovascularization (BV) and leukocyte infiltration (L). (d) The PRF gel panel with numerous keratocytes between the dense regular collagen bundles. Original enlargement; ×400, scale bar = 50 µm, hematoxylin and eosin stain.
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea; Corneal epithelium and dense regular collagen bundles. (b) Day 0 group with induced corneal ulcer showing dispersed collagen bundles. (c) The positive control group showing regenerated corneal epithelium and dense irregular collagen bundles. (d) The PRF gel group shows regenerated corneal epithelium and dense regular collagen bundles. Original enlargement; ×200, scale bar = 100m, Crossmons trichrome staining.
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea; Corneal epithelium and normal normal collagen type I. (b) Day 0 induced corneal ulcer group showing dispersed collagen type I. (c) The positive control group showing regenerated corneal epithelium and abnormal collagen types. (d) The PRF gel panel shows regenerated corneal epithelium and normal normal type I collagen. Note the new normal mature collagen stained red with the Picro-Sirius red staining technique. Original enlargement; ×200, scale bar = 100 m, Picro Sirius red spot technique.
Photomicrograph of paraffin sections in donkey cornea. (a) The negative control group shows the normal histological structure of the cornea; Corneal epithelium, clear PAS-positive Bowman's membrane and PAS-positive collagen bundles of corneal stroma. (b) 0-day induced corneal ulcer group showing distributed faint PAS-positive collagen bundles of corneal stroma. (c) The positive control group, showing regenerated keratinized corneal epithelium, poorly clear and disrupted PAS-positive Bowman's membrane, and PAS-positive collagen bundles of the corneal stroma. Note the keratin layer (arrowhead) of the regenerated corneal epithelium. (d) The PRF gel panel showing regenerated corneal epithelium, clear PAS-positive Bowman's membrane, and PAS-positive collagen bundles of the corneal stroma. Original enlargement; ×200, scale bar = 100 m, PAS staining technique.
On day 0, microscopic examination revealed epithelial loss and subepithelial stromal reaction characterized by edema and disorganized collagen fibers (Figs. 6b, 7b, 8b, 9b, 10b and 11b). In the positive control group, histopathological examination after 35 days showed re-epithelialization, stromal inflammation, vascularization, and thickening (Figs. 6c, 7c, 8c).
In contrast, the A-PRF gel group displayed re-epithelialization, stromal inflammation with normal collagen bundles, and regular keratocyte activation and proliferation (Figs. 6d, 7d, 8d). The present study demonstrated the activation and proliferation of keratocytes in the PRF gel group compared to the control group (Fig. 8a–d).
With Crossmons trichrome staining demonstrated irregular collagen bundles in the day 0-induced corneal ulcer group, dense irregular bundles in the positive control group, and dense, almost regular bundles in the PRF gel group (Fig. 9a–d). Picro Sirius red staining revealed dispersed type I collagen in the day 0-induced corneal ulcer group, abnormal collagen types in the positive control group and normal type I collagen in the PRF gel group (Fig. 10a–d).
PAS staining showed faint collagen bundles in the corneal stroma of the day 0-induced corneal ulcer group, regenerated keratinized epithelium and disrupted Bowman's membrane in the positive control group, and regenerated non-keratinized epithelium and intact Bowman's membrane in the PRF gel group (Fig. 11a–d). Overall, the histopathological changes and healing scores indicated improved healing in the PRF gel group compared to the control groups. (Table 3 provides a summary of these findings).
Various degrees and intensities of keratitis were observed in both groups. The ulcer surface area, as measured by fluorescein staining, decreased significantly over time in all groups. Specifically, the A-PRF gel group showed a significant reduction in ulcer area. Histopathological examination revealed re-epithelialization of the corneal defect and a slightly decreased subepithelial stromal inflammatory response in the treated group. Additionally, there was evidence of keratocyte activation and proliferation in the A-PRF gel group compared to the control group.
To the author's knowledge, this study is the first to investigate the effects of A-PRF gel on corneal ulcer healing in horses. Previous studies have utilized PRF in the treatment of corneal ulcers in horses32,43, dogs44,45,46 and humans47,48,49,50. PRF can be used as a clot, membrane, or plug, either directly or after compression. The supernatant can also be used in drop32 or injectable (I-PRF) form51. In this study, A-PRF was used, which was a slower and more sustained release of growth factors as compared to standard PRF52. The fibrin network of A-PRF protects the growth factors from degradation, resulting in a higher and prolonged release of growth factors over 10 days21,53.
All donkeys in the study tolerated the experiment well, with only mild signs of stress observed four days after inducing corneal ulcers. Previous studies have also reported donkeys tolerating similar eye experiments without major complications54. Throughout the study period, there were no significant changes in the clinical symptoms of the donkeys, consistent with previous research 47,54,55.
However, ophthalmic signs of inflammation were observed in all donkeys after inducing corneal ulcers, particularly after A-PRF treatment. These signs were attributed to the irritating effect of sodium hydroxide used to induce the ulcers. Corneal alkaline burns are known to trigger a strong inflammatory response characterized by cellular infiltration and the production of enzymes and cytokines56,57. Similar signs of inflammation have been observed in other studies involving donkeys58 and rabbits25,55 in donkeys after chemically inducing corneal ulcers. Ocular symptoms were also observed during PRF treatment, as PRF can cause an inflammatory response59. It is worth noting that the administration of epidermal growth factor (EGF) in horses with corneal ulcers has been associated with adverse effects such as corneal edema, vascularization, melanosis, and scarring43. Similarly, rabbits exhibited a severe inflammatory reaction after PRF membrane application as a conjunctival transplant, although the intensity of the reaction decreased over time47. Prolonged episodes of corneal epithelialization have been suggested to increase collagenase production in the corneal stroma, potentially leading to corneal perforation60.
Towards the end of the study, the donkeys treated with A-PRF gel exhibited milder ocular symptoms compared to the control group. This can be attributed to the significant re-epithelialization of the corneal defect and the prolonged release of growth factors from the gel. The conjunctivitis observed in the A-PRF gel group may be due to the temporary tarsorrhaphy used to keep the gel in place. The study showed a significant decrease in the surface area of the induced corneal ulcer over time in all animals, which is consistent with previous research in donkeys58. The inflammatory response following alkali combustion was quantified by area measurements of re-epithelialization and neovascularization61. Corneal wound healing is a complex process involving various factors, including proteinases, growth factors, cytokines, and lacrimal glands62,63,64 While PRF has been used to treat corneal ulcers and has shown potential benefits, other studies have reported adverse effects on ulcer healing43,47,48. In this study, A-PRF gel was directly applied to the ocular surface, which has been found to prevent dehydration65. Dehydration and shrinkage of the gel can affect its structural integrity, reduce growth factor content, and decrease leukocyte viability 66.
After applying Leukocyte Platelet Rich Fibrin (L-PRF), the corneal ulcer was completely sealed by the seventh day, with a whitish spot remaining until the 30th day. Although it weakened significantly by the 40th day, it was still present44. It is known that reducing the size of epithelial defects can lower the risk of infection and alleviate pain and discomfort43. Burling et al. (2000) found that re-epithelialization of corneal defects in horses started rapidly and progressed steadily within the first 5 to 7 days after surgery. However, the healing rate slowed down after this initial period43.
In the present study, all donkeys in both the control group and the A-PRF gel group showed no uptake of fluorescein dye at the end of the study, indicating successful re-epithelialization. Fluorescein dye is commonly used to diagnose corneal ulcers67, and treatment is continued until no fluorescein uptake is observed68. Although simple and indolent ulcers can be stained by fluorescein, in this study, staining was observed beneath the periphery of the corneal epithelium, suggesting detachment from the underlying stroma12. This resulted in larger surface values compared to concurrent values without staining.
To address corneal injuries, topical treatment with blood derivatives is utilized to compensate for the lack of natural angiogenesis in this avascular tissue. These blood-derived preparations contain growth factors, cytokines, and other signaling molecules that play a crucial role in cell turnover during corneal wound healing69. They can also suppress inflammation and possess antimicrobial properties70. The effectiveness of PRF in tissue regeneration is attributed to its key components, including fibrin as a supportive matrix, platelets rich in growth factors, and various types of leukocytes and stem cells that contribute to antibacterial, neovascularization, and regenerative properties71,72. The PRF membrane additionally provides mechanical support as a scaffold for cell proliferation, differentiation, and migration, which are essential for ocular surface regeneration51. Burling et al. (2000) studied the positive effects of growth factors like EGF and PDGF on the proliferation of epithelial cells and keratocytes43. Shen et al. (2011) have reported that the concentration and frequency of blood derivative administration have been found to influence the wound healing process. While the use of PRF gel is currently limited, it has shown promising results in the complete clinical healing of grade II corneal ulcers within 7 days71. However, incomplete healing was observed within 10 days for grade III ulcers, although a whitish patch remained44. In cases of severe ulcerative keratitis, PRF can be used as an alternative to corneal transplantation to preserve a significant portion of the cornea44.
In the present study, complications such as pigmentation and anterior synechia were observed in the PRF gel group, which is consistent with findings from other human studies47,48. However, the use of homologous L-PRF membrane proved effective in sealing severe corneal ulcers, including grade III ulcers with or without perforations and staphyloma44. Alkaline-induced corneal lesions often result in deep ulceration and perforation, leading to the formation of anterior synechiae and iris prolapse72. Dogs with corneal ulcers have also exhibited various clinical signs such as pain, inadequate tear film, corneal ulceration, edema, and significant neovascularization56. Alkali injuries are considered one of the most severe eye injuries, causing permanent visual impairment in one or both eyes. These injuries induce oxidative stress and inflammation in polymorphonuclear leukocytes73. The extent of inflammation following alkali burns can be measured by assessing the area of re-epithelialization and neovascularization61. Histopathological analysis revealed corneal epithelial regeneration in all donkeys in both the control and PRF gel groups. However, the control group exhibited signs of inflammation, including subepithelial coagulation, edema, leukocyte infiltration, vascular congestion, and neovascularization in the corneal stroma. In contrast, the PRF gel group only showed edema and neovascularization in the stroma. The PRF gel group also displayed numerous keratocytes between the stromal cells, resembling normal corneal histology.
PRF is known to have immune functions, including leukocyte chemotaxis and the release of cytokines such as IL-1, IL-4, IL-6, and TNF-a. It also contains anti-inflammatory cytokines like IL-474. Microscopic examination revealed minimal inflammatory features in the gel-treated group compared to the control group, which is consistent with previous studies72,75. The presence of leukocytes and cytokines in the fibrin network may play a significant role in regulating inflammation and infection at the healing site72,76.
In this study, the use of PRF gel led to the proliferation of corneal epithelium and keratocytes, but some side effects were observed. Another study by Burling et al. (2000) found that the use of EGF in different doses resulted in faster healing of corneal ulcers, but also caused side effects such as corneal edema, vascularization, melanosis, and scarring43. The A-PRF gel promotes cellular proliferation, differentiation, and migration, providing temporary mechanical support to the growing cells and releasing growth factors slowly 47,77.
The corneal regeneration rate was higher in the group treated with A-PRF gel compared to the control group, likely due to the higher concentration of growth factors present in the gel 47,76. The results of this study showed that the stromal regeneration in the A-PRF gel group resembled that of normal corneal tissue, while the control group showed thickening of the corneal stroma. Another study by Can et al. (2016) using a PRF membrane for descemetocele treatment in a human patient also resulted in the synthesis of newly formed corneal tissue similar in thickness to the surrounding healthy cornea47. By Crossmons trichrome staining, the PRF gel group exhibited dense and regular collagen bundles in the stroma, whereas the control group had irregular and scattered collagen bundles. When stained with Picro Sirius red stain in the present study, the corneas in the A-PRF gel group showed mature type I collagen bundles, similar to normal corneal histology, while the control group exhibited abnormal collagen formation47. PAS staining revealed clear and intact Bowman's membrane and collagen bundles in the PRF gel group, resembling normal corneal histology. However, the control group showed disrupted Bowman's membrane. The control group also demonstrated keratinization in the regenerated corneal epithelium, which was not observed in the PRF-treated groups. Another study reported normal epithelial regeneration with a well-organized, non-keratinized epithelium, which was not observed in the PRF-treated groups78.
The overall mechanisms of the A.PRF illustrated in the following diagram (Fig. 12):
Diagram showing the overall mechanism of the A.PRF and the role of each components of it: KGF (keratinocyte growth factor): growth and new generation of keratinocytes. PDGF (platelet derived growth factor): cell growth, new generation and repair of blood vessels, collagen production. VEGF (vascular endothelial growth factor): growth and new generation of vascular endothelial cells. EGF (epidermal growth factor): promotion of epithelial cell growth, angiogenesis, and promotion of wound healing. FGF (fibroblast growth factor): tissue repair, cell growth, collagen production. TGF.B (transforming growth factor beta.1): growth of epithelial cells, endothelial cells, promotion of wound healing.
Our findings indicate that advanced platelet-rich fibrin (A-PRF) is beneficial for the healing of corneal ulcers. The histological analysis showed that the PRF gel group had superior outcomes compared to the control group in terms of inflammation, re-epithelialization, absence of epithelial keratinization, regularity of collagen bundles, collagen type, and maturity. Additionally, the A-PRF gel group experienced fewer corneal complications during the healing process compared to the control group. These complications may be attributed to the repeated suturing of the eyelids and the irritation caused by directly applying the unfixed A-PRF gel to the cornea. Based on these results, we recommend using A-PRF gel as an alternative approach, avoiding eyelid suturing, and minimizing corneal irritation.
All data generated or analyzed during this study are included in this published article and its additional information files.
Brooks, D. E. & Matthews, A. G. Equine ophthalmology. In. Veterinary Ophthalmology (ed Gelatt, K.N.). 4th ed. 1192–1211 (Blackwell Publishing, Professional, 2007).
Nasisse, M. P. & Nelms, S. Equine ulcerative keratitis. Vet. Clin. N. Am. Equine. Pract. 8, 537–555 (1992).
Brooks, D. E. Equine ophthalmology. In Veterinary Ophthalmology (eds Brooks, D. E., Matthews, A. G.). 2nd ed. 1053–1116 (Philadelphia, 1999).
Brooks, D.E. Corneal ulceration. In Ophthalmology for the Equine Practitioner. 1st ed. 57–86 (Teton New Media, 2002).
Andrew , SA & Willis , AM Diseases of the Cornea and Sclera .In.Equine Ophthalmology (ed. Gilger, BC).1st ed.157–251 (Elsevier Saunders, 2005).
Clode, A. B. & Matthews, A. G. Diseases and surgery of the cornea. In. Equine Ophthalmology (ed. Gilger, B.C.). 2nd ed. 181–215 (Elsevier Saunders, 2011).
Johns, I. C., Baxter, K., Booler, H., Hicks, C. & Menzies-Gow, N. Conjunctival bacterial and fungal flora in healthy horses in the UK. Vet. Ophthalmol 14, 195–199 (2011).
Brown, S. I., Bloomfield, S. E. & Tam, W. The cornea-destroying enzyme of Pseudomonas aeruginosa. Invest. Ophthalmol 13, 174 (1974).
Monod, M. et al. Secreted proteases from pathogenic fungi. Int. J. Med. Microbiol 292, 405–419 (2002).
Article CAS PubMed Google Scholar
Bentley, E. Spontaneous chronic corneal epithelial defects in dog. J. Am. Anim. Hosp. Assoc. 3, 158–165 (2005).
Williams, L. B. & Pinard, C. L. Corneal ulcers in horses. Compend. Contin. Educ. Vet 35(1), E4 (2013).
Cutler, T. J. Corneal epithelial disease. Vet. Clin. N. Am. Equine Pract. 20, 319–343 (2004).
Ollivier, F. J. Medical and surgical management of melting corneal ulcers exhibiting hyperproteinase activity in the horse. Clin. Tech. Equip. Pract. 4, 50–71 (2005).
Miron, R. et al. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry?. Clin. Oral Invest. 21, 2619–2627 (2017).
Inatomi, T. et al. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am. J. Ophthalmol. 142(5), 757–764. https://doi.org/10.1016/j.ajo.2006.06.004 (2006).
Manni, L. et al. Nerve growth factor: Basic studies and possible therapeutic applications. Growth Factors. 31(4), 115–122. https://doi.org/10.3109/08977194.2013.804073 (2013).
Article CAS PubMed Google Scholar
Marquez, E. B., De Ortueta, D., Royo, S. B. & Martinez-Carpio, P. A. Epidermal growth factor receptor in corneal damage: Update and new insights from recent reports. Cutan. Ocul. Toxicol. 30(1), 7–14. https://doi.org/10.3109/15569527.2010.498398 (2011).
Article CAS PubMed Google Scholar
Anitua, E. et al. Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 93(8), e605–e614. https://doi.org/10.1111/aos.12710 (2015).
Brignole-Baudouin, F., Warnet, J. M., Barritault, D. & Baudouin, C. RGTA-based matrix therapy in severe experimental corneal lesions: Safety and efficacy studies. J. Fr. Ophtalmol. 36, 740–747. https://doi.org/10.1016/j.jfo.2013.01.012 (2013).
Article CAS PubMed Google Scholar
Guerra, M. et al. Neurotrophic keratopathy: Therapeutic approach using a novel matrix regenerating agent. J. Ocular Pharmacol. Ther. 33(9), 662–669 (2017).
Dohan Ehrenfest, D. M., De Peppo, G. M., Doglioli, P. & Sammartino, G. Slowrelease of growthfactors andthrombospondin-1 inChoukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 27, 63–69 (2009).
Article CAS PubMed Google Scholar
Bielecki, T. & Dohan Ehrenfest, D. M. Leukocyte- and platelet-rich plasma (L-PRP)/fibrin (L-PRF) in medicine—Past, present, future. Curr Pharm Biotechnol 13(7), i–ii (2012).
Al-Badran, A., Bierbaum, S. & Wolf-Brandstetter, C. Does the choice of preparation protocol for platelet-rich fibrin have consequences for healing and alveolar ridge preservation after tooth extraction? A meta-analysis. J. Oral Maxillofac. Surg. 81, 602 (2023).
Silveira, B. B. B., Teixeira, L. N., Miron, R. J. & Martinez, E. F. Effect of platelet-rich fibrin (PRF) membranes on the healing of infected skin wounds. Arch. Dermatol. Res. 315(3), 559–567 (2023).
Article CAS PubMed Google Scholar
Abd-Elkawi, M., Sharshar, A., Misk, T., Elgohary, I. & Gadallah, S. Effect of calcium carbonate nanoparticles, silver nanoparticles and advanced platelet-rich fibrin for enhancing bone healing in a rabbit model. Sci. Rep. 13(1), 15232 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Dohan Ehrenfest, D. M., Rasmusson, L. & Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 27(3), 158–167 (2009).
Article CAS PubMed Google Scholar
Choukroun , J. , Adda , F. , Schoeffler , C. & Vervelle , A. An opportunity in paro-implantology: PRF [in French].Implantology 42, 55–62 (2001).
Dohan, D.M., Choukroun, J., Diss, A., Dohan, S.L., Dohan, A.J., Mouhyi, J. & Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 101(3), 37–44 (2006).
Dohan Ehrenfest, D. M., Del Corso, M., Diss, A., Mouhyi, J. & Charrier, J. B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 81(4), 546–555 (2010).
Article CAS PubMed Google Scholar
Prakash, S. & Thakur, A. Platelet concentrates: Past, present and future. J. Maxillofac. Oral Surg. 10, 45–49 (2011).
Article PubMed PubMed Central Google Scholar
Stoppini, R. & Gilger, B. C. Equine ocular examination basic techniques. Equine Ophthalmol. 4, 1–39 (2016).
Hosny, O. H., Abd-Elkareem, M., Ali, M. M. & Ahmed, A. F. Effect of autologous serum derived from advanced platelet-rich fibrin on the healing of experimentally-induced corneal ulcer in donkeys (Equus asinus). J. Adv. Vet. Res. 12(1), 73–85 (2022).
Schultz, G. S. et al. Treatment of alkali-injured rabbit corneas with a synthetic inhibitor of matrix metalloproteinases. Invest. Ophthalmol. Vis. Sci. 33, 3325–3331 (1992).
Levinson, R. A., Paterson, C. A. & Pfister, R. R. Ascorbate acid prevents corneal ulceration and perforation following experimental alkali burns. Invest. Ophthalmol. 15, 986–993 (1976).
Öztürk, F. et al. The effect of propolis extract in experimental chemical corneal injury. Ophthalm. Res. 32, 13–18 (2000).
Ghanaati, S. et al. Advanced platelet-rich fibrin (A-PRF)—A new concept for cell based tissue engineering by means of inflammatory cells. J. Oral Implantol. 40(6), 679–689 (2014).
Petroutsos , G. , Guimaraes , R. , Giraud , J. & Pouliquen , Y. Antibiotics and corneal epithelial wound healing .Arch.Ophthalmol.Rev. 101, 1775 (1983).
Article CAS PubMed Google Scholar
Knottenbelt, D. C. & Malalana, F. Saunders Equine Formulary E-Book (Elsevier Health Sciences, 2014).
Fischer, A.H., Jacobson, K.A., Rose, J. & Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harbor. Protocol 2008; pdb.prot4986 (2008).
Aterman, K. & Norkin, S. The periodic acid—Schiff reaction. Nature 197, 1306–1306 (1963).
Article ADS CAS PubMed Google Scholar
Crossmon, G. A modification of mallorus connective tissue stain with discussion of the principle involved. Anat. Rec. 69, 33–38 (1937).
Bhutda, S. et al. Histochemical staining of collagen and identification of its subtypes by picrosirius red dye in mouse reproductive tissues. Bio-Protocol 7, e2592–e2592 (2017).
Article PubMed PubMed Central Google Scholar
Burling, K. et al. Effect of topical administration of epidermal growth factor on healing of corneal epithelial defects in horses. Am. J. Vet. Res. 61, 1150–1155 (2000).
Article CAS PubMed Google Scholar
Mishra, A. et al. Evaluation of PRP drop and L-PRF membrane for aggressive ulcerative keratitis in dogs. J. Anim. Res. 11(1), 181–185 (2021).
Pereira, V.B.S., da Silva Barbirato, D., do Lago, C.A.P. & do Egito Vasconcelos, B.C. The effect of advanced platelet-rich fibrin in tissue regeneration in reconstructive and graft surgery: Systematic review. J. Craniofac. Surg. 34(4), 1217–1221 (2023).
Demir, A., Erdikmen, D. O., Sevim, Z. T. & Altundağ, Y. The use of an autologous platelet-rich fibrin (PRF) membrane for the treatment of deep corneal ulcers in dogs. Veterinarski Arhiv 92(4), 443–458 (2022).
Can, M. E. et al. A novel technique for conjunctivoplasty in a rabbit model: Platelet-rich fibrin membrane grafting. J. Ophthalmol. 2016, 1965720 (2016).
Article PubMed PubMed Central Google Scholar
Marin-Paya , E. , Rahhal-Ortuño , M. , Aguilar-Gonzalez , M. , Fernandez-Santodomingo , AS & Garcia-Delpech , S. Use of Vivostat PRF® in Acanthamoeba keratitis .Romance.J. Ophthalmol.64(2), 222 (2020).
Ashour, S. H. et al. The effects of injectable platelet-rich fibrin and advanced-platelet rich fibrin on gingival fibroblast cell vitality, proliferation, differentiation. Tissue Eng. Regener. Med. 31, 1–12 (2023).
Nurhidayat, B. R. & Komaratih, E. Effect of platelet rich fibrin membrane on the expression of smooth muscle actin and transforming growth factor beta in corneal wound healing Pseudomonas aeruginosa keratitis patient: A literature review. Bali Med. J. 12(2), 1855–1861 (2023).
He, L., Lin, Y., Hu, X., Zhang, Y. & Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and diff erentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 108, 707–713 (2009).
Kobayashi, E. et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Invest. 20(9), 2353–2360 (2016).
Lundquist, R., Dziegiel, M. H. & Agren, M. S. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regener. 16, 356–363 (2008).
Ibrahim, A. & Ahmed, A. F. The impact of surgical excision of the orbital lacrimal gland on the aqueous tear production and ocular surface health in donkeys (Equus asinus). J. Equine Vet. Sci. 97, 103344 (2021).
Gunay, C. et al. Evaluation of autologous serum eyedrops for the treatment of experimentally induced corneal alcali burns. Revue Méd. Vét. 166(3–4), 63–71 (2015).
Christmas, R. Management of chemical burns of the canine cornea. Can. Vet. J. 32(10), 608–612 (1991).
CAS PubMed PubMed Central Google Scholar
Imanishi, J. et al. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 19, 113–129 (2000).
Article CAS PubMed Google Scholar
Sobhy, M. Evaluation and Comparison of the Effect of Honey, Aloe Vera Gel and Cod Liver Oil on Healing of Experimentally Induced Corneal Ulcer in Donkeys: An Experimental Study. Master Thesis, Faculty of Veterinary Medicine, Assiut University (2016).
Aricioglu, C., Dolanmaz, D., Esen, A., Isik, K. & Avunduk, M. C. Histological evaluation of effectiveness of platelet-rich fibrin on healing of sinus membrane perforations: A preclinical animal study. J. Cranio-Maxillofac. Surg. 45(8), 1150–1157 (2017).
De Aracena Del Cid, RM & De Espinosa Escoriaza, IM Subconjunctival application of regenerative factor-rich plasma for the treatment of ocular alkali burns.Eur.J. Ophthalmol.19(6), 909–915 (2009).
Conners, M. S. et al. Alkali burn-induced synthesis of inflammatory eicosanoids in rabbit corneal epithelium. Invest. Ophthalmol. Vis. Sci. 38, 1963–1971 (1997).
Schultz, G.S. Modulation of corneal wound healing. In Cornea (eds Krachmer, J.H., Mannis, M.J., Holland, E.J.). 1st ed. 183–196 (Mosby, 1997).
Sack, R. A. et al. Towards a closed eye model of the preocular tear layer. Prog. Retin. Eye. Res. 19, 649–668 (2000).
Article CAS PubMed Google Scholar
Sivak, J. M. & Fini, M. E. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye. Res. 21, 1–14 (2002).
Article CAS PubMed Google Scholar
Aroca, S., Keglevich, T., Barbieri, B., Gera, I. & Etienne, D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J. Periodontol. 80, 244–252 (2009).
Article CAS PubMed Google Scholar
Dohan Ehrenfest, D. M. et al. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 108, 341–352 (2009).
Gilger, B.C. & Davidson, M.G. How to prepare for ocular surgery in the standing horse. In Proceedings of the American Association for Equine Practice. 266–271 (2002).
Williams, L. B. & Pinard, C. L. Corneal ulcers in horses. Compend. Contin. Educ. Vet. 35(1), E4 (2013).
Klenkler, B., Sheardown, H. & Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 5, 228–239 (2007).
Alio, J. L., Arnalich-Montiel, F. & Rodriguez, A. E. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr. Pharm. Biotechnol. 13, 1257–1265 (2012).
Article CAS PubMed Google Scholar
Shen, E. P. et al. Comparison of corneal epitheliotrophic capacity among different human blood-derived preparations. Cornea 30(2), 208–214 (2011).
Donzis, P. B. & Mondino, P. J. Management of noninfectious corneal ulcers. Surv. Ophthalmol. 32(2), 94–110 (1987).
Article CAS PubMed Google Scholar
Bashkaran, K. et al. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: Experimental animal study. BMC Complement. Altern. Med. 11, 1 (2011).
Dohan, D. M. et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 101(3), e45–e50 (2006).
Simonpieri, A., Del Corso, M., Sammartino, G. & DohanEhrenfest, D. M. The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: A new grafting protocol. Implant Dent. 18(2), 102–111 (2009).
Kokdere, N. N., Baykul, T. & Findik, Y. The use of platelet-rich fibrin (PRF) and PRFmixed particulated autogenous bone graft in the treatment of bone defects: an experimental and histomorphometrical study. Dent. Res. J. 12, 418–424 (2015).
Park, J. W., Hwang, S. R. & Yoon, I. S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 22, E1259 (2017).
Freire, V. et al. Corneal wound healing promoted by 3 blood derivatives: An in vitro and in vivo comparative study. Cornea 33, 614–620 (2014).
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Assiut University, Assiut, 71526, Egypt
Omar H. Hosny, Magda M. Ali & Ahmed F. Ahmed
Department of Cell and Tissues, Faculty of Veterinary Medicine, Assiut University, Assiut, 71526, Egypt
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
O.H. operated the experiment, followed up the animals, collected and analyzed the data, did the statistical testes and wrote the manuscript; M.A. performed the histological examination of the cornea, wrote the histological data in the manuscript and revised the manuscript; M.M. cooperated in the performed the experiment and revised the manuscript; A.F. supervise the work, cooperated in the performed the experiment, wrote part in the manuscript and revised the manuscript. All authors read and approved the final manuscript.
Correspondence to Omar H. Hosny.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Hosny, O.H., Abd-Elkareem, M., Ali, M.M. et al. Advanced platelet-rich fibrin promotes healing of induced corneal ulcer in donkeys (Equus asinus). Sci Rep 13, 21824 (2023). https://doi.org/10.1038/s41598-023-48933-5
DOI: https://doi.org/10.1038/s41598-023-48933-5
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Scientific Reports (Sci Rep) ISSN 2045-2322 (online)
Prp Tubes Fda Approved Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.